Unveiling the First-Ever X-Ray of a Single Atom: A Scientific Breakthrough
Written on
Chapter 1: A New Era in Atomic Imaging
The field of atomic imaging has taken a significant leap with the advent of advanced techniques. The emergence of the electron microscope in 1931 marked the beginning of this journey, allowing researchers to observe individual atoms for the first time. This milestone was further enhanced by the introduction of the scanning tunneling microscope (STM) in 1981, which provided atomic resolution through the measurement of quantum tunneling currents.
Atomic imaging has evolved remarkably since its inception, paving the way for new discoveries.
In 1985, the atomic force microscope (AFM) was developed, utilizing a sharp tip to measure forces and yielding high-resolution images of atomic structures. The innovation continued with the introduction of scanning transmission electron microscopy (STEM) in 2009, which combined electron microscopy with compositional analysis to identify different elements in a sample.
Recent technological advancements, such as aberration-corrected electron microscopy and helium ion microscopy, have significantly deepened our understanding of atomic structures and chemical processes, facilitating breakthroughs in materials science and nanotechnology.
Section 1.1: The Journey to the First X-Ray of a Single Atom
In a landmark achievement in 2008, physicists captured an image of a single hydrogen atom using an electron microscope. This was followed in 2013 by the use of a "quantum microscope," which allowed scientists to observe electron orbitals directly. Most recently, researchers from Ohio University, Argonne National Laboratory, and the University of Illinois-Chicago have successfully captured the first X-ray image of a single atom.
This pivotal research integrates synchrotron X-rays with quantum tunneling, paving the way for future experiments that could simultaneously characterize the elemental and chemical properties of materials at the ultimate single-atom level.
Section 1.2: Quantum Tunneling and Its Implications
Utilizing a finely pointed conducting probe, researchers employed a technique known as "quantum tunneling" to interact with the electrons of the material under study. When the probe is brought extremely close (about half a nanometer), the precise position of an electron becomes uncertain, causing it to disperse across the space between the probe and the material. Measuring the resulting current allows scientists to determine the state of the atom.
The first video titled "How Scientists take world first X-ray of a single atom" explains the groundbreaking techniques and their implications in detail.
Chapter 2: The SX-STM Technique
The innovative combination of synchrotron X-ray scanning tunneling microscopy (SX-STM) plays a crucial role in this research. By stimulating the sample with amplified X-radiation, the resultant photoelectrons are collected via a needle-like detector. This technique has shown remarkable potential, as evidenced by previous studies that utilized SX-STM to manipulate single molecules.

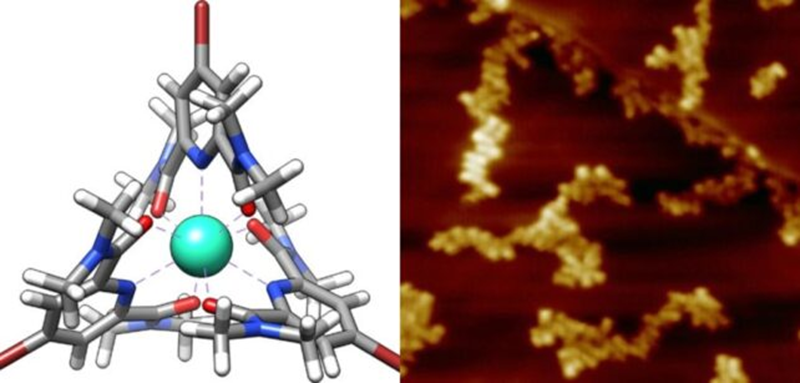
In their latest investigation, researchers focused on the properties of an individual iron atom. They created distinct supramolecular assemblies featuring iron and terbium ions enclosed within a ligand ring. The configuration included one iron atom linked to six rubidium atoms via terpyridine ligands, while terbium, oxygen, and bromine were connected using pyridine-2,6-dicarboxamide ligands.
The second video titled "Revolution in Physics: First-Ever X-Ray of a Single Atom" dives deeper into the scientific breakthroughs and their potential impact.
The researchers then employed the SX-STM technique on these samples. Interestingly, the light detected differed from the initial light directed at the sample. Specific wavelengths absorbed by electrons in the atomic core created darker lines in the X-ray spectrum. Analysis revealed that these lines corresponded to wavelengths absorbed by the iron and terbium atoms.
Section 2.1: Detecting Quantum Phenomena
By examining the absorption spectra, researchers could ascertain the chemical states of the atoms. Notably, for the iron atom, the X-ray signal was only detectable when the probe tip was precisely positioned directly above the iron atom at an extremely close distance. This confirmed detection in the tunneling regime, highlighting the involvement of quantum phenomena, and suggesting significant implications for quantum mechanics studies. The complete findings are documented in the Journal of Nature.
For more insightful stories like this, follow Faisal Khan on Medium and stay updated with the latest in science and technology.