Exploring the Biological Foundations of Psychedelics
Written on
Psychedelics are potent psychoactive agents that significantly alter perception and interaction with environmental stimuli, leading to shifts in consciousness. Research indicates that classic psychedelics, such as psilocybin, LSD, DMT, and ayahuasca, show promise in treating various stress-related conditions.
These substances may provide antidepressant, anxiolytic, and cognitive-enhancing effects, potentially through biological mechanisms akin to traditional antidepressants or rapid-acting agents like ketamine. A key hypothesis is that they facilitate neuroplasticity in the brain, an essential factor in their therapeutic efficacy.
In my initial article on Medium, I explored why psychedelics are effective in treating mental health issues, emphasizing the impact of neuroplasticity on entrenched behavioral and cognitive patterns common in mental disorders. I also speculated on how psychedelics can disrupt these persistent thought patterns in just one or two treatments, which you can read more about here.
In this piece, we delve deeper into the biological mechanisms of psychedelic-induced neuroplasticity, referencing a recent review published in Frontiers in Psychiatry by researchers from the Maastricht University's Department of Neuropsychology and Psychopharmacology.
Introduction
Classic serotonergic psychedelics derive their name from their primary action on serotonin (5-HT) receptors in the brain, significantly affecting perception and cognition. A glance at their molecular structures reveals similarities to serotonin, indicating their strong binding affinity to 5-HT receptors.
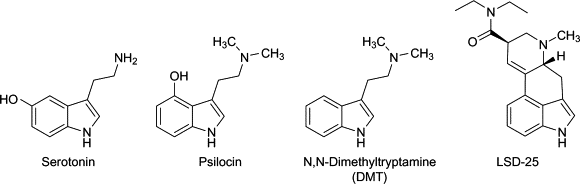
In addition to their interaction with serotonin receptors, psychedelics also bind to dopamine and adrenergic receptors, as detailed below. Molecules can function as antagonists or agonists; antagonists block responses, while agonists activate receptors, with psychedelics primarily acting as agonists or partial agonists.
Both psilocybin and its active form, psilocin, demonstrate binding affinity for:
- A variety of serotonin receptors (5-HT1A/B/D/E, 2B, 5, 6, 7), particularly the 5-HT2A receptor.
- The dopamine D3 receptor.
LSD shows affinity for:
- 5-HT1A/D, 2A/B/C, and 5-HT6.
- Dopamine D1 and D2, with overlapping agonism for the 5-HT2A and D2 receptors.
- The ?-adrenergic receptors.
DMT binds to:
- 5-HT1A/D and 5-HT6 receptors, with a high affinity for the 5-HT2A receptor.
- The sigma-1 receptor.
Neuroplasticity
Research indicates that psychedelics can induce lasting changes in behavior and cognition, suggesting a biological adaptation beyond the substance's immediate effects. Neuroplasticity plays a crucial role in these enduring changes.
Neuroplasticity is the brain's lifelong capability to reorganize itself, involving structural changes and adaptations in neuronal communication. This adaptability allows the brain to form new connections and modify existing ones in response to experiences. While neuroplasticity can occur throughout life, it is particularly pronounced from birth until about age 25.
Molecular Level
At the molecular level, neuroplastic changes occur through signaling pathways—intracellular cascades that transmit signals from cell membrane receptors to the nucleus, influencing gene expression.
Activation of these pathways can occur via:
- Ca²? influx (substance movement into the cell) through depolarization.
- N-methyl-D-aspartate (NMDA) receptor activation.
Upon activation, various signaling pathways are stimulated, including:
- Ca²?/calmodulin-dependent protein kinase (CaMK2).
- Extracellular regulated kinase 1/2 (ERK1/2).
- Mitogen-activated protein kinase (MAPK).
- Brain-derived neurotrophic factor/tropomyosin receptor kinase B (BDNF/TrkB).
Within the nucleus, two proteins modulate gene transcription and protein synthesis related to neuroplasticity:
- The cyclic AMP-responsive element-binding protein (CREB).
- The nuclear factor kappa B protein complex (NF-kB).
Immediate early genes (IEGs) are rapidly expressed during neuronal activity and are crucial for synaptic plasticity.
Cellular Level
Neuroplasticity can lead to either structural or functional changes at the cellular level, which can be categorized as follows:
- Neuronal plasticity.
- Dendritic plasticity.
- Synaptic plasticity.
Neuronal plasticity involves the growth and reorganization of neuronal networks, a process called neurogenesis that unfolds through five phases:
- Proliferation.
- Differentiation.
- Survival.
- Migration.
- Maturation.
During proliferation, progenitor cells arise in the hippocampal subgranular zone and evolve into dentate granule neurons. Surviving cells then migrate, mature, and integrate into the hippocampal network.
Dendritic plasticity refers to changes in the number and complexity of dendritic spines, which are linked to synaptic strength. The formation of dendritic spines is driven by neurotransmitter release, such as GABA or glutamate.
Synaptic plasticity pertains to alterations in neuron structure and function, which is crucial for learning and memory. This process can manifest as long-term potentiation (LTP) or long-term depression (LTD) and is primarily regulated by BDNF levels, which tend to be lower in individuals with depression, anxiety, and addiction.
> “In summary, neurobiological changes, particularly enhanced neuroplasticity, are hypothesized to explain psychedelics' therapeutic effects.”
The authors stated.
> “Understanding the biological pathways of psychedelics’ acute and lasting effects is vital to realize their full therapeutic potential. Although psychedelics lack established therapeutic applications in psychiatry, promising initial findings warrant further exploration and provide insights into the biological basis of psychiatric disorders.”
Findings
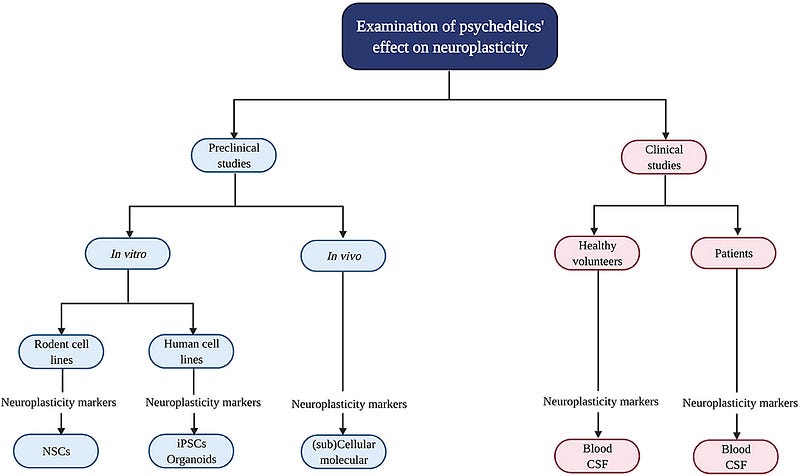
The review authors analyzed preclinical and clinical studies to discern the immediate, short-term, and long-term effects of serotonergic psychedelics on molecular and cellular neuroplasticity. Four notable findings emerged:
The first finding highlights dose discrepancies between preclinical and clinical studies, which are crucial when translating animal research to human applications.
> “The results indicate that the highest doses used in clinical studies are comparable to the lowest doses of LSD in preclinical research, underscoring a critical factor in translating findings.”
The second finding concerns gender differences in psychedelic response, potentially linked to sex-specific neuroplasticity changes.
> “The female hormone estrogen shows antidepressant effects by stimulating BDNF and synaptic plasticity, differing between genders.”
The third finding emphasizes the need for direct measurement of BDNF in clinical studies.
> “All clinical studies reported peripheral BDNF levels, which only indirectly reflect brain activity; cerebrospinal fluid (CSF) BDNF levels would provide a clearer picture.”
The fourth finding pertains to the small sample sizes in some in vivo studies, which can limit the reliability of conclusions.
> “This prevalent issue in biological research necessitates careful justification for the number of animals used, which can affect the statistical power of findings.”
Neuroplasticity and 5-HT2A Receptor Activation
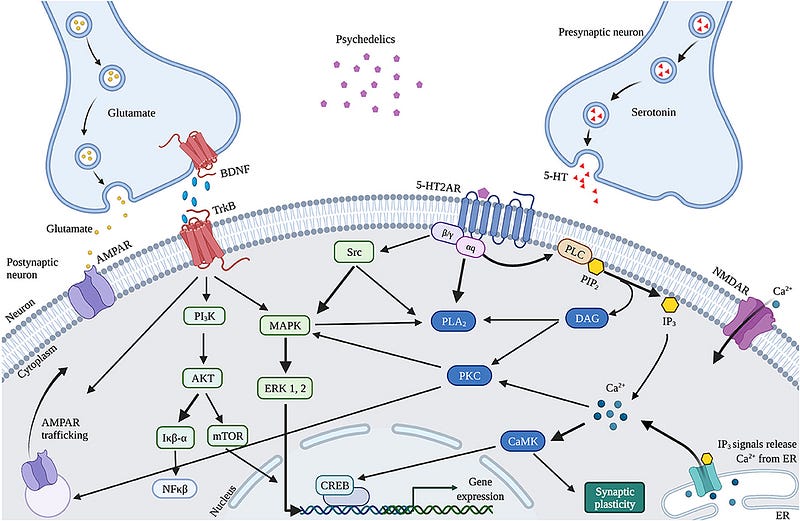
Research indicates that psychedelic-induced neuroplasticity changes are linked to the activation of 5-HT2A receptors, affecting both serotonergic and glutamatergic systems. Psychedelics predominantly activate 5-HT2A receptors on glutamatergic pyramidal cells in the cortex, stimulating intracellular signaling pathways such as phospholipase C (PLC), phospholipase A (PLA), and Src—pathways believed to underlie the hallucinogenic effects of these substances.
These intracellular pathways promote synaptic plasticity through:
- Ca²? influx.
- Glutamate influx.
> “Increased glutamate release in the cortex can further enhance synaptic plasticity via AMPA receptors on pyramidal neurons, increasing the density of AMPA receptors and leading to greater glutamate and BDNF release.”
Indirectly, psychedelics may influence neuroplasticity through the expression of BDNF and other plasticity-related genes and proteins, which include:
- LSD and ayahuasca upregulate cortical BDNF mRNA.
- IEGs play roles in synaptogenesis and plasticity.
- Many IEGs encode proteins involved in specific signaling cascades.
Some examples of IEGs are:
- Arc, located at dendrites, involved in cytoskeletal rearrangements.
- Egr2, associated with NMDA receptors.
- Sgk, which promotes cell survival.
- Neuron-derived orphan receptor 1 (Nor1; NR4A3), important for long-term potentiation.
Sigma-1 Receptor
Variations in neuroplasticity effects among psychedelics may be attributed to differences in receptor affinity, as they are not solely 5-HT2A receptor agonists. DMT, for instance, also strongly binds to the sigma-1 (S1) receptor, which is prominent in the hippocampus and stimulates synaptic plasticity.
- Activation of the S1 receptor by DMT aids synaptic plasticity alongside 5-HT2A receptor modulation, likely contributing to ayahuasca’s antidepressant effects.
- LSD indirectly activates the S1 receptor through dehydroepiandrosterone (DHEA), promoting synaptic plasticity and neurogenesis.
- S1 receptor activation by SSRIs enhances BDNF expression.
> “These findings support the hypothesis that psychedelics stimulate neuroplasticity through mechanisms akin to SSRIs.”
Ketamine
Ketamine functions as an NMDA receptor antagonist, with its antidepressant properties also believed to result from increased synaptic plasticity and BDNF levels.
- Ketamine blocks NMDA receptors on glutamatergic neurons in the cortex.
- This action relieves the inhibition on BDNF translation by deactivating eEF2 kinase, subsequently raising BDNF levels.
Additionally,
- Ketamine may block NMDA receptors on GABA interneurons, increasing glutamate release.
- This activation stimulates AMPA receptors on glutamatergic cells, elevating BDNF and glutamate levels in the cortex.
- Both psychedelics and ketamine activate cortical AMPA receptors, enhancing BDNF and synaptic efficacy.
Conclusion
This review is the first to elucidate the rapid antidepressant and cognitive effects of psychedelics by examining the molecular and cellular alterations associated with neuroplasticity. A deeper understanding of the biological mechanisms underlying serotonergic psychedelics underscores the necessity for continued scientific inquiry in this area.
These substances offer potential benefits not only for those with psychiatric disorders but also for healthy individuals, enhancing social and cognitive skills, including empathy and creativity, as well as overall well-being.
> “Further investigation is crucial to clarify the specific (intra)cellular mechanisms activated by various psychedelics, their long-term effects, and their association with altered behavior. Current findings support research into psychedelics as treatments for psychopathologies.”
Thank you for your interest in science! Please feel free to leave any questions, comments, or suggestions for future articles in the comment section.
If you'd like to support:
- If you're not a Medium member yet, consider using my referral link so I can receive a portion of your fees from Medium at no extra cost to you.
- Subscribe to my Newsletter to receive the best tutorials, research, education, and scientifically-based tools for daily life directly in your inbox.
While you’re here, check out one of my other articles.
Safety of Group Psychedelic Sessions Confirmed by Psilocybin Clinical Trial
DMT: The Spirit Molecule That Keeps Amazing Us