All Physics Areas in Your Kitchen: Understanding Kinetics
Written on
Exploring the Physics of the Kitchen
The kitchen, often regarded as the heart of the home, hosts a myriad of physical phenomena, many of which go unnoticed. In our previous discussion, we delved into the realm of Mechanics. This time, we will uncover the invisible actions occurring at the microscopic level within our culinary spaces.
The Connection Between Humans and Tools
Humans have a natural fascination with tools that extend our capabilities. Just as a telephone amplifies our hearing and glasses enhance our vision, kitchen utensils serve as extensions of our physical abilities. For instance, a spoon acts as an extension of our hand, while a fork mimics the functionality of our fingers. In this article, we will examine the unseen physics that operates in our kitchens.
The Kinetic Theory of Matter
With advancements in physics, we often hear about the seven states of matter, yet most of us are familiar with the three fundamental states: solid, liquid, and gas, with plasma sometimes included. These states, often referred to as phases, are crucial to understanding the world around us.
James Clerk Maxwell made significant contributions to physics, particularly through his electromagnetic theory, which bridged electricity and magnetism, and his kinetic theory of gases. This theory elucidated the origins of gas pressure and laid the groundwork for statistical physics developed by Boltzmann and Gibbs.
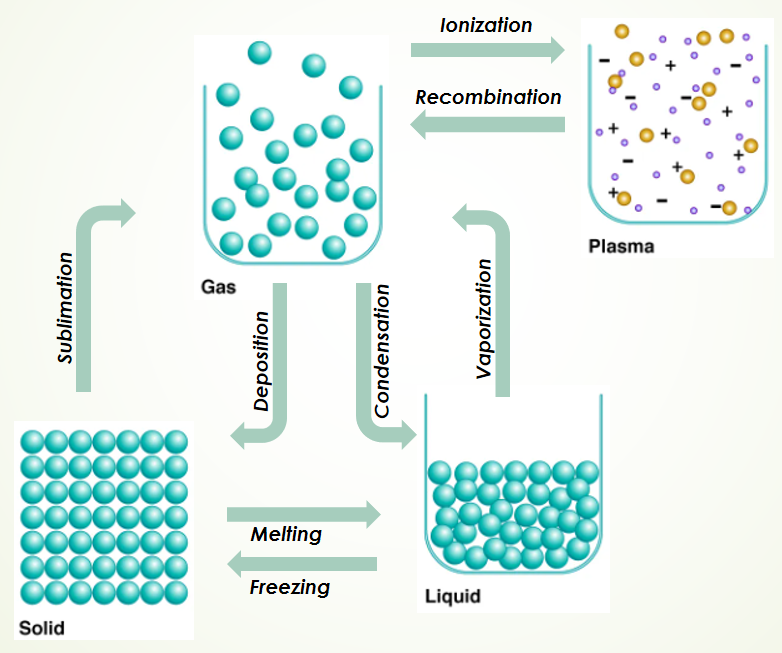
According to the kinetic theory, the states of matter are linked to the motion of their particles. Transitioning between these states occurs when we alter the temperature, leading to what is termed thermal agitation.

In solids, molecules are tightly bound in a fixed arrangement, only able to vibrate. Liquids allow for greater movement, with molecules loosely packed. Gases, however, present molecules that are far apart and occupy the full volume of their container.
Understanding Temperature and Molecular Movement
Each molecule within a substance possesses a distinct energy level. Boltzmann and Gibbs defined temperature as a measure of the average energy associated with molecular motion. Thus, an increase in molecular agitation corresponds to a rise in average kinetic energy, indicating a higher temperature.
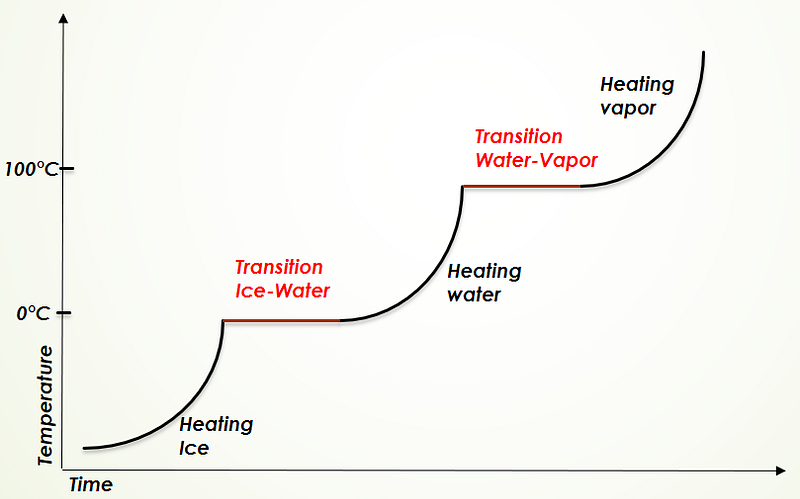
The concept of absolute zero represents a state where molecular motion ceases entirely, forming the basis for the Kelvin temperature scale. Notably, when a substance changes from one phase to another, its temperature remains constant, as illustrated by the straight lines in the accompanying diagram.
For example, heating an ice cube from below zero degrees Celsius will raise its temperature until it reaches zero, where it remains until all ice has melted. Once melted, the temperature will rise again until reaching 100°C, at which point water turns to vapor. The energy required to facilitate these phase changes is known as Latent Heat.
The Unique Case of Water
Water is exceptional as it naturally exists in all three fundamental states on Earth due to specific temperature and pressure conditions. This unique quality emphasizes water's significance in both natural and life sciences.
When two bodies at different temperatures come into contact, heat transfers from the warmer object to the cooler one. The capacity of a substance to absorb heat is termed heat capacity. For example, ice requires 2 Joules per gram per degree to increase its temperature. Therefore, a 10-gram ice cube needs 300 Joules to raise its temperature from -15°C to 0°C.
To melt the ice, additional energy, known as the heat of fusion, is required. For water, this is about 333 Joules per gram, meaning a 10-gram ice cube needs 3330 Joules to transform into liquid water.
When we exit a bath, water droplets evaporate, drawing energy from our skin and causing the sensation of goosebumps.
The Role of Pressure in Phase Changes
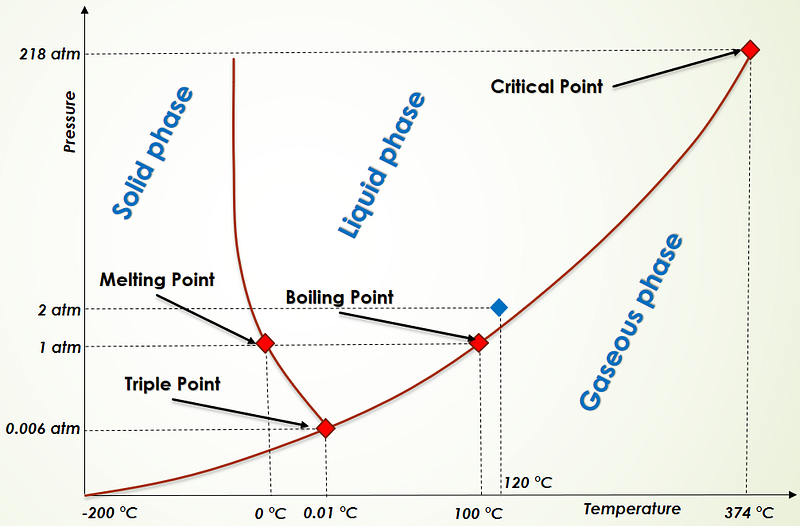
A well-known phase diagram illustrates the relationship between temperature and pressure. This diagram reveals three primary regions corresponding to the three basic states of matter.
At 1 atmosphere of pressure, cooling water below 0°C results in ice, while heating it above 100°C produces vapor. However, under increased pressure, water can remain liquid beyond 100°C, as seen in pressure cookers.
Denis Papin, in 1679, sought to cook food faster by heating water above 100°C using pressure, leading to the invention of the digester.
The modern pressure cooker, developed by the LESCURE brothers in 1953, cooks food at temperatures below 120°C and pressures exceeding 1.8 atm. Safety mechanisms are essential to prevent accidents from valve malfunctions or sudden openings.
Boiling Water Dynamics
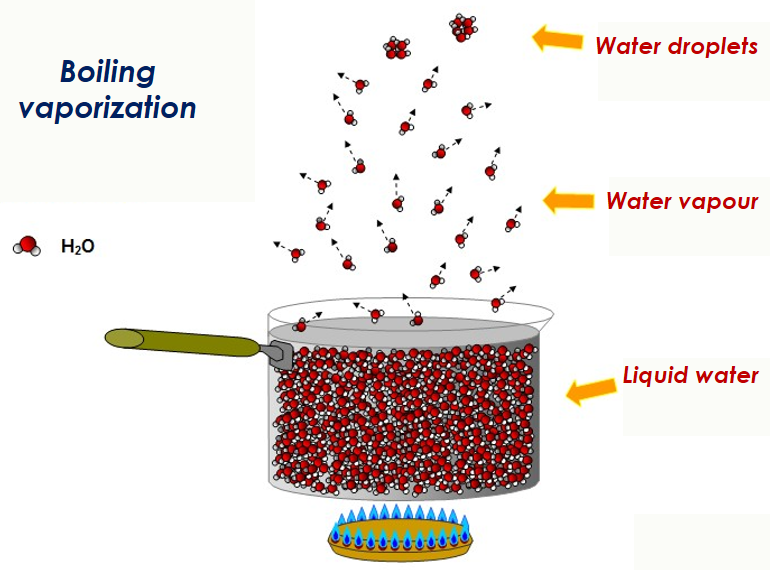
In the context of boiling water in an open container, the water molecules at the surface experience pressure only from the molecules beneath, as atmospheric pressure is effectively ignored. Heating water past 100°C leads to vapor bubble formation within the liquid as boiling occurs.

Kinetics is a vast field of physics, but we will conclude here to avoid transforming this discussion into a full physics lesson. In our next installment, we will venture into thermodynamics in the kitchen to explore the cooking process and the refrigeration chain.