generate a new title here, between 50 to 60 characters long
Written on
Chapter 1: The Journey to Einsteinium
The 20th century marked a significant era in scientific advancements, particularly with the discovery of nuclear fission. Among the elements unearthed from atomic explosions was einsteinium. Nearly seven decades after its initial discovery, scientists have finally amassed enough einsteinium to begin fundamental research.
While it was understood that einsteinium (atomic number 99) should exist on the periodic table, it remained unidentified until 1952, following the United States' detonation of the “Ivy Mike” hydrogen bomb in the Marshall Islands. Unfortunately, the element's extreme instability meant that it decayed before extensive studies could be conducted. This pattern persisted for almost 70 years—until now.
Thanks to advances in technology, we no longer rely on nuclear detonations for einsteinium production. Researchers have established a consistent, albeit limited, source of this element at Oak Ridge National Laboratory’s High Flux Isotope Reactor, which primarily produces heavy elements like californium (atomic number 98). While californium is a valuable neutron source, its production process also yields some einsteinium. Typically, einsteinium is found mixed with other materials, rapidly decaying into berkelium and subsequently into californium.
Researchers at Lawrence Berkeley National Laboratory successfully isolated a minuscule sample of pure einsteinium, measuring just 200 nanograms. Previously, it was believed that a minimum of 1,000 nanograms was necessary for analysis. The team conducted X-ray absorption spectroscopy on the sample, uncovering an unexpected blueshift in emitted light, which indicates a shortening of wavelength. Contrarily, they anticipated a redshift, suggesting that the bond distances in einsteinium are shorter than previously estimated based on neighboring elements on the periodic table.
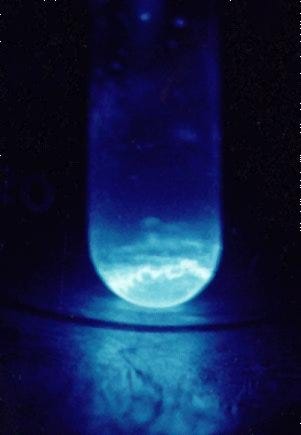
However, the ongoing coronavirus pandemic significantly hampered the experiment, as it has affected many scientific endeavors over the past year. The research team was unable to finalize X-ray diffraction tests that would have provided deeper insights into the electron and molecular bond structures of einsteinium before the laboratory was shut down. By the time they regained access, too much of the sample had decayed into californium—einsteinium decays at a rate of approximately 3.3 percent daily. Consequently, the contaminated sample was no longer fit for further analysis.
The silver lining is that more einsteinium will be produced at the reactor every few months, laying the groundwork for future exploration of this enigmatic element.
In the first video, "Scientists Create, Capture Mysterious 99th Element, 'Einsteinium'," you can learn more about the background and significance of einsteinium in modern science.
The second video, "Scientists Discovered New Elements Beyond The Periodic Table," explores the exciting discoveries surrounding new elements, including einsteinium and its implications for chemistry and physics.
Chapter 2: The Future of Einsteinium Research
As we continue to gather more einsteinium in the coming months, the potential for groundbreaking research in this field becomes increasingly promising. The insights gained from these studies could significantly enhance our understanding of the periodic table and the characteristics of heavy elements.